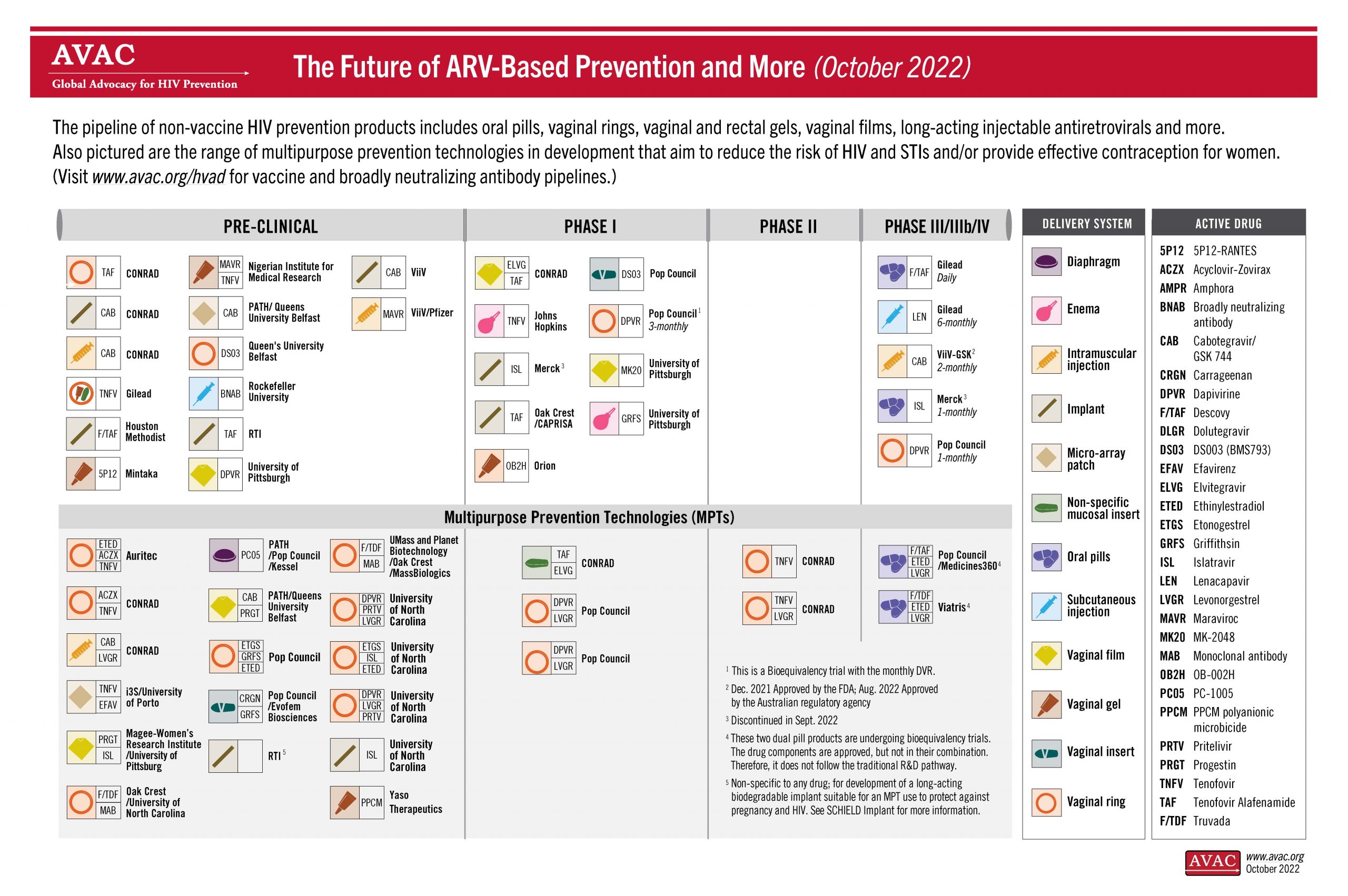
Research Pipeline
The pipeline of non-vaccine HIV prevention products includes pills, vaginal rings, vaginal and rectal gels, vaginal films, long-acting injectable antiretrovirals and more. Also pictured are the range of multipurpose prevention technologies in development that aim to reduce the risk of HIV and STIs and/or provide effective contraception for women.
For a comprehensive overview of all planned, ongoing, and completed clinical trials for new PrEP products, visit AVAC’s HIV Prevention Research and Development Database.
For detailed information on technologies and pharmaceutical compounds in development, visit the Long-Acting Therapeutics Patents and Licenses Database (LAPaL). LAPaL is a free online database that provides information on long-acting therapeutics that could have impact in resource-constrained settings. It includes technical and intellectual property information on these technologies as well as an interactive visualisation tool to navigate the pipeline progress.
Click here for information on vaccine and broadly neutralizing antibody pipelines.