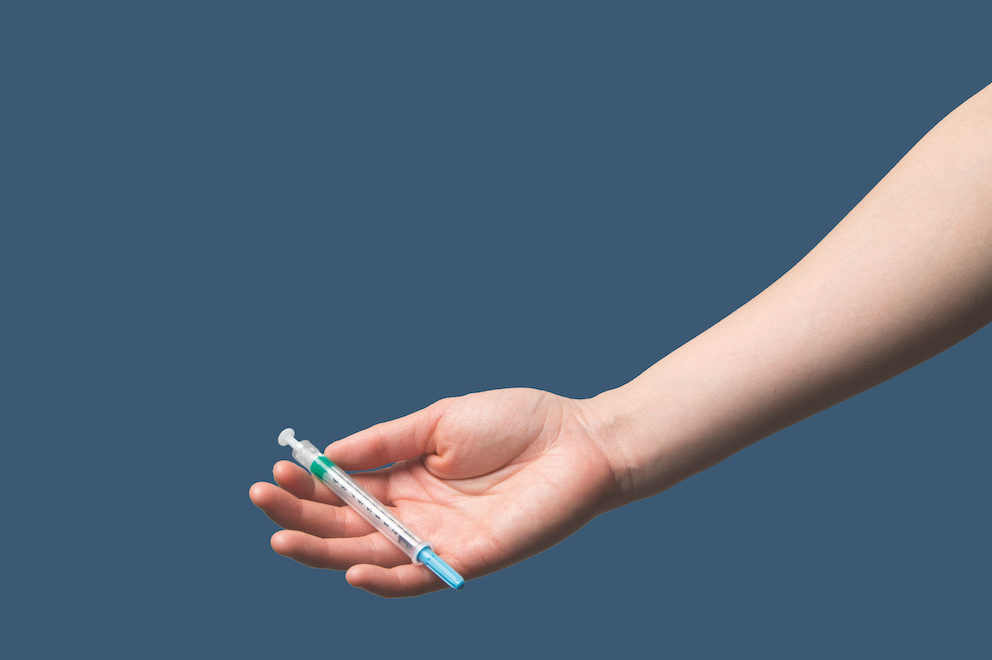
Injectable PrEP uses antiretroviral drugs administered through injection for longer-acting, systemic protection against HIV acquisition. In general, systemic prevention strategies protect against HIV regardless of the route of infection, with drugs remaining active throughout the body for much longer periods of time compared to localized topical options, such as the PrEP Ring.
One injectable has been approved as PrEP using cabotegravir (CAB). CAB for PrEP was added to the toolkit of approved products for HIV prevention in December of 2021 with approval by the US Food and Drug Administration. Click on our dedicated page on injectable CAB for PrEP for information on the evidence for efficacy, rollout considerations, associated advocacy and more.
Other injectable forms of PrEP are in development. See our page on injectable lenacapavir for information on the clinical trials investigating the efficacy of lenecapavir and go to our page on upstream R&D for a look at injectables in the pipeline.