Data by Country
Curious about the state of PrEP across the world? Find the Global PrEP Tracker, interactive maps, and where to access PrEP here.
Explore by Country
- Albania
- Antigua & Barbuda
- Argentina
- Armenia
- Australia
- Austria
- Azerbaijan
- Bahamas
- Bangladesh
- Barbados
- Belarus
- Belgium
- Belize
- Benin
- Botswana
- Brazil
- Bulgaria
- Burkina Faso
- Burundi
- Cambodia
- Cameroon
- Canada
- Chile
- China
- Colombia
- Costa Rica
- Côte d'Ivoire
- Croatia
- Cuba
- Cyprus
- Czechia
- Denmark
- Djibouti
- Dominica
- Dominican Republic
- DRC
- Ecuador
- El Salvador
- England
- Eritrea
- Estonia
- Eswatini
- Ethiopia
- Finland
- France
- French Guiana
- Gambia
- Georgia
- Germany
- Ghana
- Greece
- Grenada
- Guatemala
- Guyana
- Haiti
- Honduras
- Hungary
- Iceland
- India
- Indonesia
- Iran
- Ireland
- Israel
- Italy
- Jamaica
- Japan
- Kazakhstan
- Kenya
- Kyrgyzstan
- Lao PDR
- Latvia
- Lebanon
- Lesotho
- Liberia
- Liechtenstein
- Lithuania
- Luxembourg
- Madagascar
- Malawi
- Malaysia
- Maldives
- Mali
- Malta
- Mexico
- Moldova
- Mongolia
- Morocco
- Mozambique
- Myanmar
- Namibia
- Nepal
- Netherlands
- New Zealand
- Nigeria
- North Macedonia
- Northern Ireland
- Norway
- Pakistan
- Panama
- Papua New Guinea
- Paraguay
- Peru
- Philippines
- Poland
- Portugal
- Romania
- Rwanda
- Saint Lucia
- Scotland
- Senegal
- Serbia
- Seychelles
- Sierra Leone
- Singapore
- Slovakia
- Slovenia
- South Africa
- South Korea
- South Sudan
- Spain
- Sri Lanka
- Sweden
- Switzerland
- Taiwan
- Tajikistan
- Tanzania
- Thailand
- Togo
- Uganda
- Ukraine
- United States
- Uruguay
- Uzbekistan
- Vietnam
- Wales
- Zambia
- Zimbabwe
Global PrEP Tracker
Use this dynamic tool to see global trends by geography, track factors such as use of different delivery models, and get key updates.
Explore the Data
This Excel document provides a sortable database with detailed information on the status of PrEP approval and delivery by product and by country.
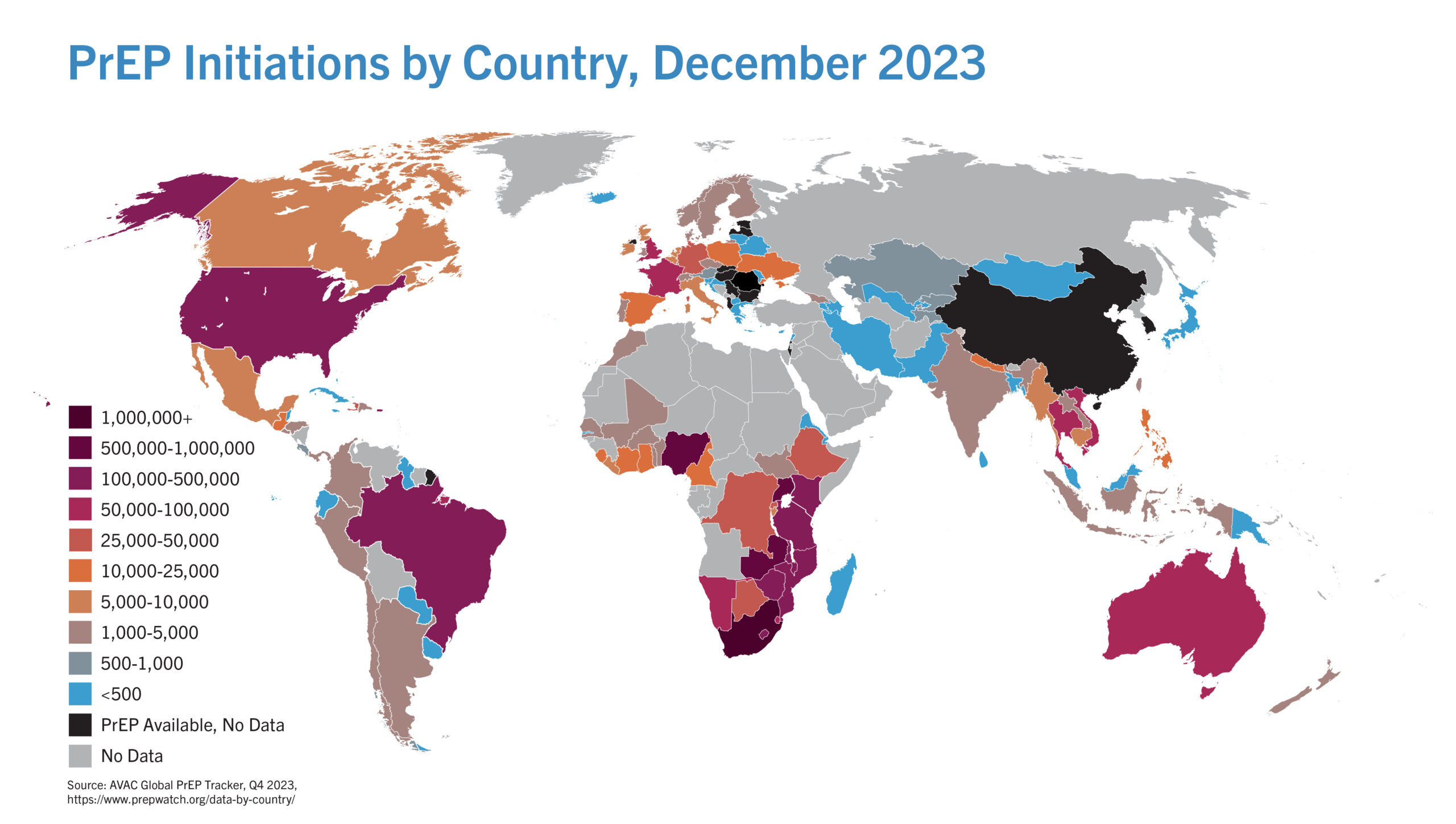
Snapshot: PrEP Initiations by Country
AVAC’s quarterly surveys of ongoing oral PrEP demonstration and implementation projects, combined with data collected from manufacturers and government agencies inform this graphic on PrEP initiations around the globe.
Explore Publications
A library of publications and presentations featuring data from the PrEP Tracker.
Click here for links to publications that are citing data from the PrEP Tracker.