Prevention Market Manager Project
Established in 2016, The Prevention Market Manager (PMM) was a comprehensive, collaborative project to accelerate the introduction of HIV prevention products, to effectively reach those who need prevention most. It was funded by the Gates Foundation and was a six year effort in partnership with The Clinton Health Access Initiative (CHAI). The Biomedical Prevention Implementation Collaborative (BioPIC) is extending this work and creating platforms for broad stakeholder coordination.
Oral PrEP rolled out slowly. This project examined why, and what should happen at every level to ensure that the next life-saving interventions scale up efficiently, reach those who need it, and bend the curve of the epidemic. The lessons from this work have been ground breaking.
Newly approved interventions typically become widely available in wealthy countries within a few years time. But scaling up new options in lower and middle-income countries lags for years, even decades, with devastating effects on global health, individual lives, and the global effort to end the epidemic.
The PMM project developed insights, recommendations, and innovative approaches that cross the continuum of research to rollout, and lays a foundation for better delivery, better uptake and better outcomes.
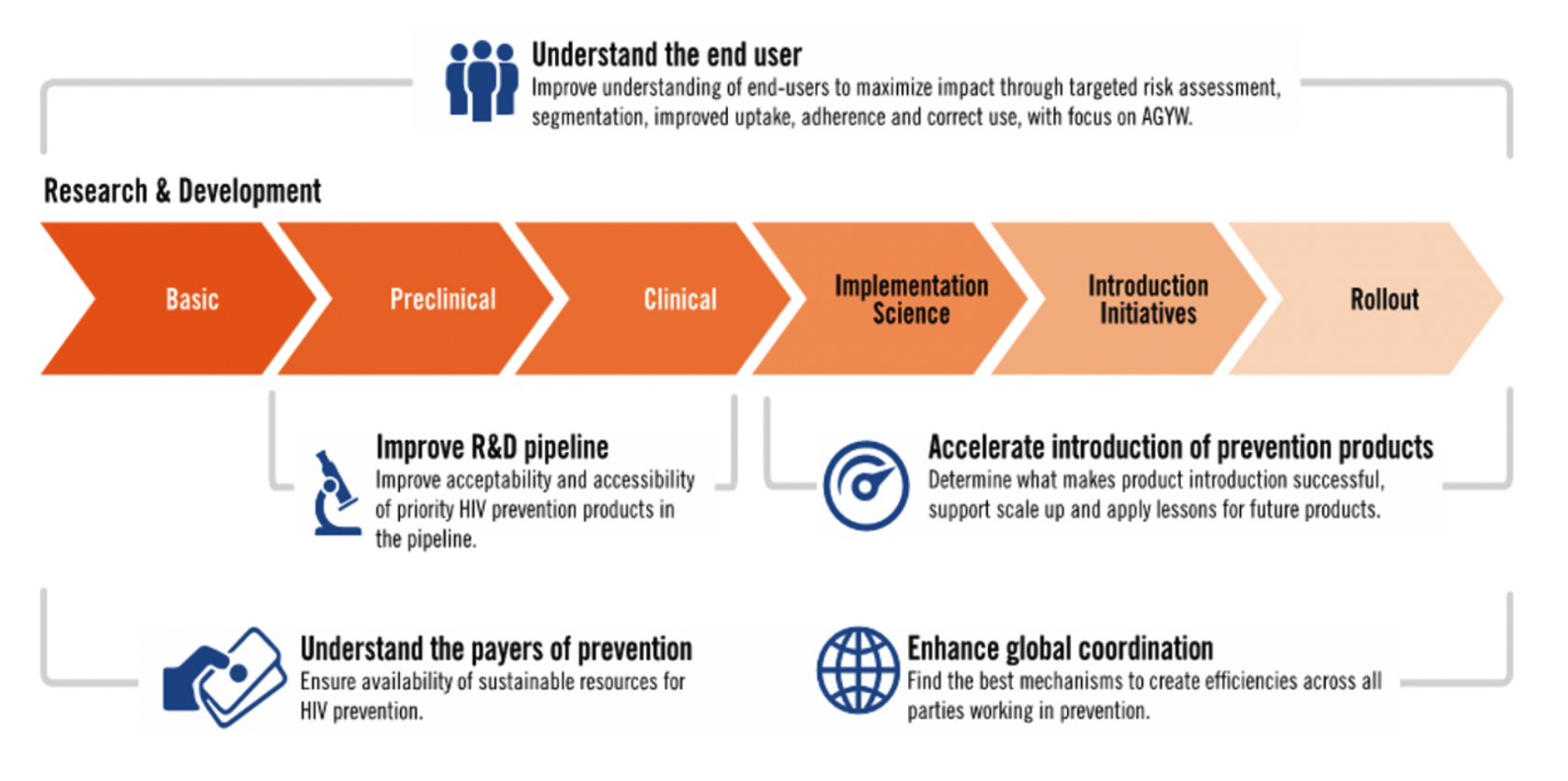
Smarter Rollout Today, Faster Rollout Tomorrow
Prevention Market Manager Themes
Three major themes: people, programs and products, describe the scope of this work and its achievements.
People: PMM initiatives put the users of products at the center of engagement across the continuum of research to rollout and yielded ground-breaking insights for product development, program design, demand creation and uptake.
Programs: Based on findings and recommendations from the PMM project, stakeholders and country partners are pursuing innovation in program design, including: integrating services for HIV and sexual and reproductive health; new metrics for effective use of HIV prevention interventions; de-medicalized and flexible options to deliver products and services; more ambitious targets and increased resources for scaling up access while expanding and improving the support for continued use.
Products: Oral PrEP, the Dapivirine Vaginal Ring (DVR), long-acting injectable cabotegravir (CAB-LA), the dual prevention pill and other products in earlier phases of development each offer unique benefits and challenges. Turning these options into choices is essential. The effort requires understanding the needs and preferences of users. Ministries of health need reliable data on costs and return on investment. Implementers benefit from a platform to share lessons, evidence and information.The Biomedical Prevention Implementation Collaborative (BioPIC), established under the PMM project, is the first effort to date focused on comprehensive coordination to address these critical areas.
Resources
Getting Rollout Right: Lessons from Oral PrEP
This report and accompanying issue briefs highlight lessons learned from oral PrEP rollout and offer practical guidance related to monitoring and evaluation, generating demand, improving delivery and reframing risk. Click here for issue briefs and the full report.
An HCD Approach to Breaking the Cycle of Transmission
PMM integrated multiple methods from human centered design to conceptualize a comprehensive picture of the journey to HIV prevention for adolescent girls and young women. Key findings are captured in short issue briefs (below) and a PowerPoint offers a full summary of the findings.
Issues Briefs:
- Rethink HIV prevention
- Re-Prioritize resources for impact
- Refine goals to incentivize better outcomes
- Promote segmentation to improve effectiveness
- Align design efforts to AGYW needs
- Ensure programme relevance
- Deploy Relationship Workshop
- Leverage this work to amplify impact
Global PrEP Tracker
A dynamic map exploring global trends in PrEP uptake.
Redefining Success With Oral PrEP: A call for better metrics
These resources offer an analysis of current and still-need metrics for measuring the impact of PrEP. The think tank, report and executive summary reflect a partnership with PMM and Jhpiego.
Evaluating, Scaling up and Enhancing Strategies for Supporting PrEP Continuation and Effective Use
This report, co-authored with Jhpiego, offers recommendations for modifying indicators for defining and measuring ongoing PrEP use.
BioPIC: Adaptable Product Introduction Framework
This report frames activities and priorities for an efficient and coordinated approach to product introduction.
Key HIV/SRH Materials and Findings
- Integration of HIV prevention and SRH services in Zimbabwe
- Integration of HIV Prevention and Sexual Reproductive Health Services in Kenya
- Integration of HIV Prevention and Sexual and Reproductive Health in the Era of Anti-retroviral-based Prevention: Findings from assessments in Kenya, Malawi and Zimbabwe
- Catalyzing Action on HIV/SRH Integration: Lessons from Kenya, Malawi, and Zimbabwe to spur investment
Visit here for more resources.
HIV Prevention Market Manager Project (PMM) Summary Powerpoint
PMM analyzed lessons from all facets of the oral PrEP experience to pave the way for faster development and introduction of the next generation of prevention strategies. This slide deck provides an overview of the project scope, findings and next steps.
AVAC’s Related Projects to Accelerate Product Innovation and Availability
This page highlights related projects with AVAC and partners to speed the ethical development and rollout of HIV prevention strategies.