Injectable Cabotegravir (CAB) for PrEP is highly effective at protecting people from HIV. Below are details on its distinct characteristics, approval history, availability, safety and effectiveness. Evidence and resources to learn more are also provided.
The Basics
- An injection with the antiretroviral (ARV) cabotegravir (CAB) given every two months.
- Intramuscular injection in the buttocks.
- Developed by ViiV Healthcare.
- First approved in 2021 by the US Food and Drug Administration.
- Approved in 50+ countries, but not yet widely available outside of implementation studies.
- Can be used by adults and adolescents who weigh at least 35 kg (77 lb) and are at risk of getting HIV through sex.
- Brand name Apretude.
- Generics currently in development but won’t be available until at least 2027.
- ViiV has granted a voluntary license to the Medicines Patent Pool.
- An oral version is also available though it is not widely used.
Effectiveness
- Highly effective if injections taken on time.
- The second injection must be taken one month after the first; after that, must be taken every two months to confer protection.
- Protection begins approximately one week after the first injection.
- May help improve adherence because it is longer-acting and may be more discreet than daily pills.
- Does not prevent any sexually transmitted infections other than HIV.
- Does not prevent pregnancy.
Safety
- Found to be safe and effective for cisgender men and women and transgender women; additional data collection is ongoing in transgender men and non-binary individuals.
- Found to be safe and effective for adolescents, and eligibility for use is based on weight (over 35 kg or 77 lbs) rather than age.
- Initial data suggests that feminizing gender affirming hormone therapy (GAHT) does not reduce the efficacy of CAB for PrEP. More data is needed to confirm if CAB for PrEP impacts the effects of feminizing GAHT and to understand the interactions between CAB for PrEP and masculinizing GAHT.
- Initial data suggests that CAB for PrEP is well-tolerated by pregnant and lactating people (PLP), with no heightened risk of congenital abnormalities, though more data is needed. For more information, see notes from BioPIC’s Think Tank on CAB for PrEP and PLP.
- Side effects are similar to oral PrEP, including nausea, abdominal cramps, and headache, and users may also experience injection site pain, swelling, or redness. These side effects generally decrease over time.
- CAB for PrEP is from a class of ARVs called Integrase Strand Transfer Inhibitors (INSTI), the same class of drugs as a common first-line HIV treatment, Dolutegravir (DTG). There is a small risk of developing INSTI resistance if a user with recently acquired HIV starts CAB for PrEP during the window period when they are not yet testing positive, necessitating second-line treatment.
- Depending on the type of HIV test used, it can take from 14 days to three months from the time of transmission for a test to detect HIV.
- The WHO recommends that people initiating and continuing CAB for PrEP use a country’s national HIV testing algorithm, which can include rapid diagnostic tests. For more information, see notes from BioPIC’s Think Tank on CAB for PrEP and HIV testing.
- CAB for PrEP remains in the bloodstream for up to a year, though after two months it is no longer at a level high enough to confer protection-this period is called the “pharmacokinetic tail.” If a user acquires HIV during the tail, they may develop INSTI resistance, and/or face delays in HIV diagnosis as the residual CAB for PrEP keeps the virus at a level below detection.
Where Can I Access CAB for PrEP?
To see where CAB for PrEP is currently approved and where approval is pending, visit our Country Planning for Product Introduction Matrix. To find a provider near you, see our PrEP Access page. At this time, CAB for PrEP is not as widely available as oral PrEP, and is only available in a small number of locations and via implementation studies.
Evidence and Resources
- HIV Prevention Trials Network (HPTN) 083 and 084 Phase III clinical trials established the effectiveness of CAB for PrEP. In the trials, participants were randomised to take either CAB for PrEP or oral PrEP (TDF/FTC).
- HPTN 083 enrolled 4,570 cisgender gay and bisexual men and trans women who have sex with men in Argentina, Brazil, Peru, South Africa, Thailand, the US, and Vietnam. Researchers found risk of HIV reduced by 66 percent compared to those taking oral PrEP.
- HPTN 084 enrolled 3,224 cisgender women in Botswana, Eswatini, Kenya, Malawi, South Africa, Uganda and Zimbabwe, and found risk of HIV reduced by 89 percent compared to those taking oral PrEP.
- Additional research is now ongoing to determine best practices for providing CAB For PrEP in real world conditions, while also investigating safety and efficacy in groups that were not included in the original trials, such as pregnant and lactating people. Click here for details of these studies. And click here for an overview of key questions these studies are seeking to answer, and what we’ve learned so far.
- Visit AVAC’s Global PrEP Tracker for the latest updates on PrEP uptake by country, including CAB for PrEP.
- The Biomedical Prevention Implementation Collaborative (BioPIC) hosts regular Think Tanks with implementers, donors, civil society, and technical experts to address key questions relating to introduction and scale-up of CAB for PrEP. Click here for Think Tank notes, which contain the latest CAB for PrEP insights.
- The Coalition to Accelerate Access to Long-Acting PrEP brings together donors, civil society, ministries of health, implementation science think tanks and partner organizations to ensure an accelerated, equitable, sustainable, and collaborative approach to optimizing access to new long-acting PrEP options, starting with CAB for PrEP. Click here for Coalition resources.
- WHO’s Guidelines on Long-Acting Injectable Cabotegravir for HIV Prevention provide considerations to support managers, policy makers, researchers, health workers, communities, and other stakeholders in the implementation of projects and programs for injectable CAB for PrEP. It also outlines critical research gaps.
- Looking to roll out CAB for PrEP in your country? RISE’s CAB for PrEP Training Toolkit can be used to train healthcare providers to deliver CAB for PrEP.
- For more CAB for PrEP resources, see our resource library.
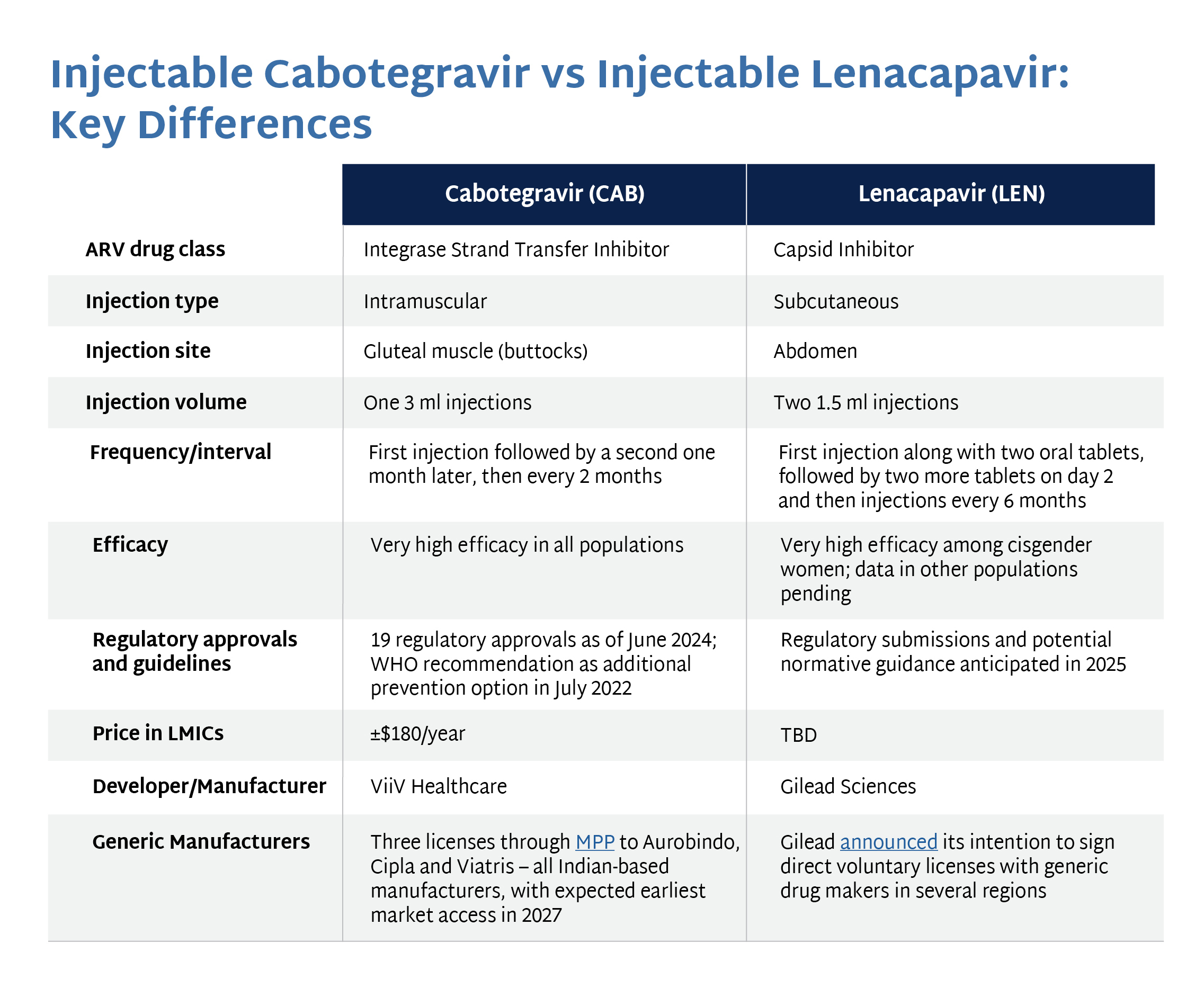
The graphic below compares cabotegravir with another injectable PrEP product, lenacapavir. Click the image to enlarge.