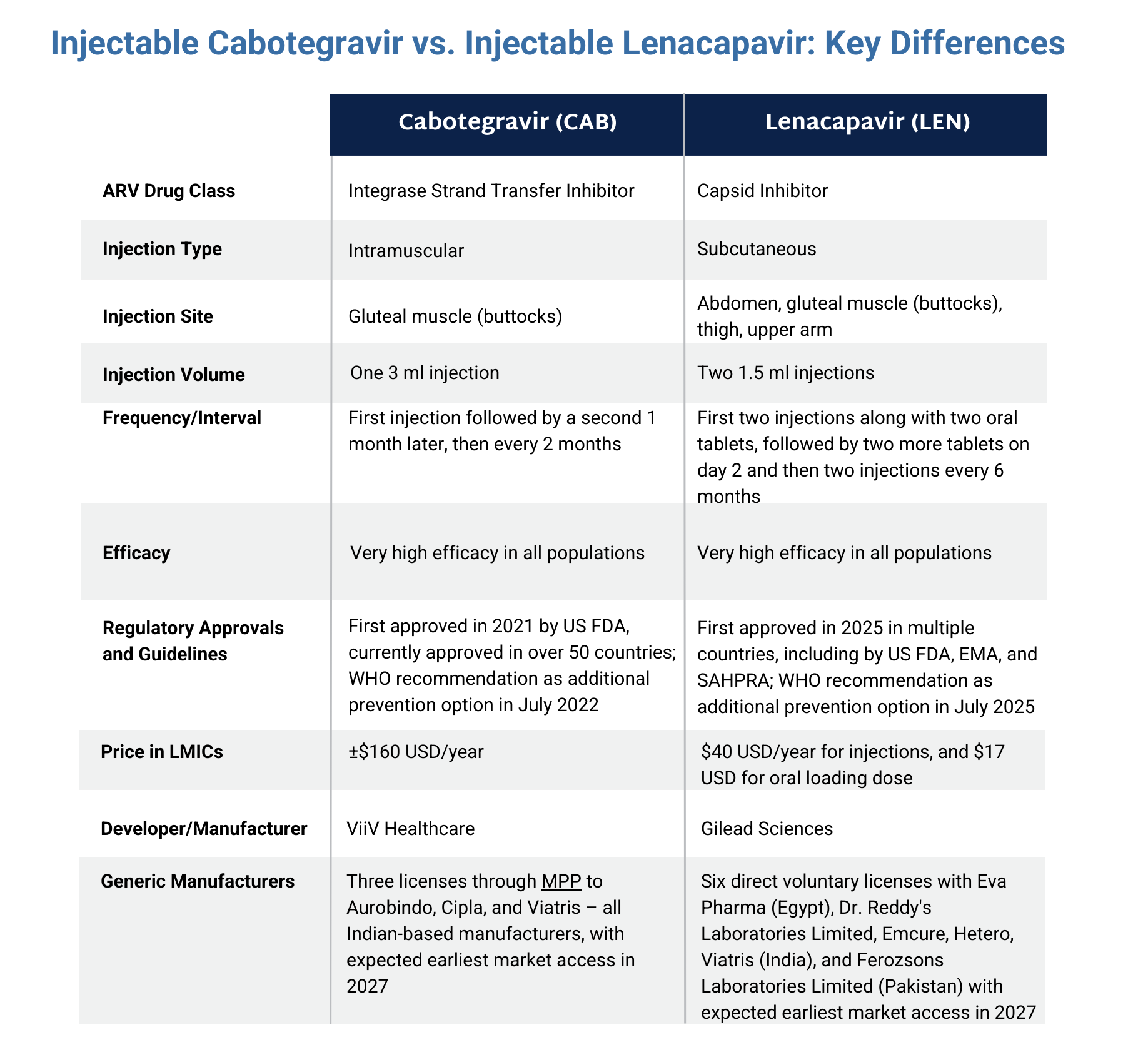
Injectable lenacapavir (LEN) for PrEP is a new antiretroviral (ARV) drug that is highly effective at preventing HIV. Below are details on its distinct characteristics, ongoing Phase II and III clinical trials, approval status by country, as well as resources to learn more.
The Basics
- Two subcutaneous injections given on the same visit in the abdomen every six months.
- The first visit also involves taking two pills with the first set of injections followed by two more pills the following day. This is called the oral loading dose.
- Developed by Gilead.
- Recommended and prequalified by WHO.
- First approved in 2025 in multiple countries, including by the US Food and Drug Administration, the European Medicines Agency, and the South African Health Products Regulatory Authority as pre-exposure prophylaxis (PrEP). Scroll down for an interactive approval map or click here for a full list of countries where LEN for PrEP is approved and where approvals are pending.
- Can be used by adults and adolescents who weigh at least 35 kg (77 lb) and are at risk of getting HIV through sex.
- Brand names include Yeztugo (USA) and Yeytuo (Europe).
- Generics currently in development and likely to become available in 2027.
- In September 2025, the Gates Foundation and Unitaid announced partnerships with two generics manufactures to make injectable LEN for PrEP available at a price of $40 USD per person per year in up to 120 countries from 2027.
- A one-year version of LEN for PrEP is also under investigation. Early results have been positive, and a Phase III clinical trial launched in 2025, expected to run through 2028.
Effectiveness
- Shown to be highly effective at preventing HIV in two large Phase III clinical trials, PURPOSE 1 and PURPOSE 2. Early results from PURPOSE 1 showed no HIV infections in the LEN for PrEP arm, and early results from PURPOSE 2 showed only two infections in the LEN for PrEP arm.
- Three smaller Phase II clinical trials- PURPOSE 3, PURPOSE 4, and PURPOSE 5- are investigating safety and efficacy in populations not included in PURPOSE 1 and 2.
- If administered as directed, with two injections and two pills on day one, and two pills on day two, then protection begins on day two. If the oral loading dose is not taken, protection may not start for at least a month.
- May help improve adherence because it is longer-acting and may be more discreet than daily pills.
- Does not prevent any sexually transmitted infections other than HIV.
- Does not prevent pregnancy.
Safety
- Found to be safe and effective for cisgender and transgender men and women.
- Found to be safe and effective for adolescents, and eligibility for use is based on weight (over 35 kg or 77 lbs) rather than age.
- Data has shown no significant interactions between LEN and gender affirming hormone therapy.
- Can be safely used by people who are pregnant or lactating.
- The most common side effects are injection site reactions, including pain, swelling, or redness, and small lumps, or nodules, under the skin where the injection was given. Some users may also experience nausea.
- LEN for PrEP is from a new class of ARVs called capsid inhibitors, and no other ARVs from this class are currently used routinely for prevention or treatment. Because of this, and the rarity of breakthrough infections, resistance to LEN is unlikely to be a significant concern.
- Due to drug interactions, people taking some common medications for high cholesterol, erectile disfunction, or tuberculosis who want to use LEN may require dosage adjustments.
The Trials
PURPOSE 1
- Conducted among approximately 5,000 cisgender women in South Africa and Uganda.
- Testing the efficacy of both LEN for PrEP and the daily pill emtricitabine/tenofovir alafenamide (F/TAF) in preventing HIV.
- In June 2024, the trial was unblinded after meeting its primary endpoint of superiority to oral PrEP (TDF/FTC) and background HIV incidence.
- Scheduled to run until July 2027.
- The first Phase III clinical trial to include pregnant and lactating people from the start—which could make it easier to get approval for use in this population if found to be effective and safe.
- Read a summary here and the full results from the New England Journal of Medicine here.
PURPOSE 2
- Conducted among 3,000 men who have sex with men, gay men, transgender men, transgender women, and gender non-binary people in Argentina, Brazil, Mexico, Peru, Puerto Rico, South Africa, Thailand, and the USA.
- In September 2024, the trial was unblinded after meeting its primary endpoint of superiority to oral PrEP (TDF/FTC) and background HIV incidence.
- Scheduled to run until April 2027.
- Read a summary here and the full results from the New England Journal of Medicine here.
PURPOSE 3
- Conducted among 250 cisgender women in the USA.
- Scheduled to run until January 2028.
PURPOSE 4
- Conducted among 250 people who inject drugs in the USA.
- Scheduled to run until July 2027.
PURPOSE 5
- Conducted among 262 men who have sex with men, gay men, transgender men, transgender women, and gender non-binary people in France and the United Kingdom.
- Scheduled to run until July 2029.
PURPOSE 365
- Investigating a new 12 month formulation of LEN for PrEP, administered intramuscularly.
- Conducted among 300 people with an indication for PrEP in the USA.
- Scheduled to run until September 2028.
Where can I access LEN for PrEP?
Outside of the USA, LEN for PrEP is not yet available outside of clinical trials, though multiple countries are currently preparing to introduce LEN in early 2026- click here to see where early LEN introduction will take place. To see where LEN for PrEP is currently approved and where approval is pending, see the graphic below.
Further Resources
Reports and Guides
- Getting LEN Rollout Right: Resources and tools to support LEN introduction
- WHO Guidelines on Lenacapavir for HIV Prevention
- The WHO and Jhpiego Provider Training Toolkit on Use of Oral and Long-Acting HIV Pre-Exposure Prophylaxis — includes clinical training resources for LEN
- Key Considerations for LEN Rollout — drawing on lessons from Getting PrEP Rollout Right this Time: Lessons from the Field
- The Gears of Lenacapavir for PrEP Rollout — this report provides a framework for accelerated and equitable introduction of injectable lenacapavir (LEN) for PrEP, including outlining priority actions for different stakeholder groups.
- From Clinical Trial Efficacy to Public Health Impact: A plan for accelerating access to injectable lenacapavir for PrEP — this plan provides a comprehensive view of all the moving parts for lenacapavir for PrEP introduction and identifies priority actions and actors responsible for ensuring time is not wasted and opportunity not squandered.
- The Lens on LEN — AVAC’s primer on lenacapavir
- Standard Operating Procedure (SOP) for Injectable Lenacapavir as Pre-exposure Prophylaxis– an adaptable SOP to support the development and adoption of national SOPs that align with WHO LEN recommendations and guidance
Infographics
- Tracking Lenacapavir Rollout — interactive graphics outlining immediate next steps on pathways to access and impact, key actors responsible for them, timelines for completion, and important progress updates
- LEN for PrEP Supply in Early Adopter Countries
- An Overview of LEN for PrEP Trials
- Lenacapavir for PrEP Regulatory Approvals Map
- Where we are with LEN for PrEP
- Moving a Product to the Real World
- LEN Generics: Can we go faster?
Understanding Trial Results
- An Overview of LEN for PrEP Trials
- AVAC’s press release on the PURPOSE 1 trial interim results
- Gilead’s press release on the PURPOSE 1 trial interim results
- AVAC’s press release on the PURPOSE 2 trial interim results
- Gilead’s press release on the PURPOSE 2 trial interim results
- Summary of PURPOSE 1 trial and results
Webinars
- The Scientific Journey of Lenacapavir: From basic science to clinical development to impact — his webinar explored how US support from NIH for basic science and South Africa’s clinical research infrastructure made possible the development of lenacapavir
Access
The graphic below compares lenacapavir with another injectable PrEP product, cabotegravir. Click here to download the graphic.