USAID Microbicide Product Introduction Initiative (MPii)
USAID Microbicide Product Introduction Initiative (MPii)
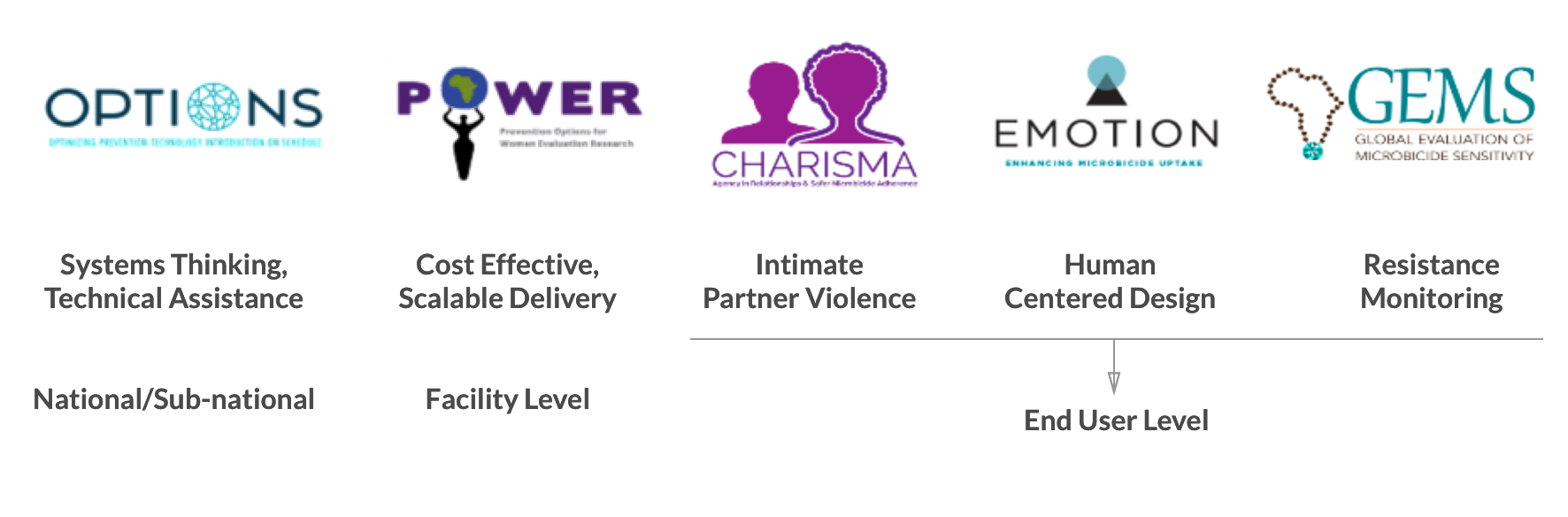
The US Agency for International Development’s (USAID) Office of HIV/AIDS is supported five interconnected projects on ARV-based HIV prevention product introduction and access, the Microbicide Product Introduction Initiative (MPii) agreements, that run from 2015-2020.
MPii Tools & Resources: Available here.
-
OPTIONS – Optimizing Prevention Technology Introduction On Schedule
Who: Core partners — FHI 360, Wits RHI, AVAC
Primary partners — Avenir Health, FSG, LVCT Health, LSHTM, McCann Health, Pangaea
What: Provide targeted support to expedite and sustain access to ARV-based HIV prevention products in countries and among populations where most needed.
Objectives: The project aims to develop evidence-based business cases and coordinated investment strategies for introduction; support country level regulatory approval, policy development, program planning, and marketing and implementation strategies; facilitate and conducting implementation science; and provide technical assistance and support for health systems strengthening with rapid use of data to identify and address implementation bottlenecks throughout the value chain.
Countries: Kenya, South Africa, Zimbabwe
-
POWER – Prevention Options for Women Evaluation Research
Who: Core partner — University of Washington
Primary partners — Desmond Tutu HIV Foundation, Kenya Medical Research Institute (KEMRI),
Wits Reproductive Health and HIV Institute (WRHI), Carnegie Mellon University , Massachusetts General Hospital (MGH), RTI International
What: Develop cost-effective and scaleable models for implementation of ARV-based HIV prevention products for women.
Objectives: The project aims to conduct formative research among African women and healthcare providers, focusing on motivators and obstacles for initiation of and adherence to microbicides and PrEP, to inform development of effective communications, decision tools, and delivery strategies that meet women’s needs and are integrated with established programs, including regular HIV testing; to establish open cohorts of women for delivery of microbicides and PrEP; pilot optimized and scalable microbicide and PrEP adherence support and delivery strategies; analyze cost-effectiveness and modeling delivery approaches; and translate successful approaches to other programmatic settings.
Countries: Kenya, South Africa
-
CHARISMA – Community Health Clinical Model for Agency in Relationships and Safer Microbicide Adherence
Who: Core partner — RTI International
Primary partners — Wits RHI,
FHI 360, Sonke Gender Justice, University of Washington
What: Increase women’s agency to safely and consistently use oral PrEP and vaginal microbicides; improve male partner communication and support; and reduce harmful gender norms and intimate partner violence (IPV).
Objectives: To identify improved approaches to measure and address the beneficial impacts and social harms—particularly IPV—of PrEP and microbicide use; to develop and test the CHARISMA intervention designed to increase women’s agency to consistently and safely use PrEP and microbicides and mitigate IPV; and to disseminate knowledge generated and promote uptake of evidence into oral PrEP programs.
Countries: South Africa
-
GEMS – Global Evaluation of Microbicide Sensitivity
Who: Core partner — University of Pittsburgh
Primary partners — FHI 360, Lancet Laboratories, UCL, Statistical Center for HIV/AIDS Research and Prevention (SCHARP)
What: Inform policies and define programmatic considerations related to use of ARV-based HIV prevention products and risk of resistance.
Objectives: The project aims to conduct research to better characterize risk of resistance with topical ARV-based microbicides and PrEP agents and the possible effects on future HIV treatment options; model and analyze potential public health harms, benefits, and costs of different intervals and requirements for HIV testing for users of microbicides in resource-constrained settings; develop and evaluating evidence-based policy recommendations for the frequency of HIV testing and resistance monitoring; and monitor seroconverters in ARV-based prevention programs for resistance.
Countries: Kenya, South Africa, Zimbabwe
-
EMOTION – Enhancing Microbicide Uptake in High-Risk End Users
Who: Core partner — CONRAD
Primary Partners — IDEO, Abt Associates, CAPRISA, RTI International, Matchboxology, Instant Grass, MatCH Research
What: Increase uptake and correct and consistent use of ARV-based HIV prevention products by women at high risk of HIV infection using an end-user centered strategy
Objectives: The project aims to conduct a human-centered design (HCD) study; implement HCD-based changes in microbicide/PrEP products and messaging; quantify the user experience of accessing and adhering to microbicide/PrEP products in a socio-behavioral study with objective biomarkers of adherence; and develop an introduction package and a campaign for marketing, distribution, and outreach for microbicide/PrEP products that are desirable to use and enhance health and partnerships.
Countries: Kenya, South Africa, Zimbabwe
