Injectable cabotegravir for PrEP (CAB for PrEP) is an antiretroviral drug developed by ViiV Healthcare and formulated to be administered by injection once every two months. Cabotegravir was previously approved in several countries for treatment, in combination with another injectable antiretroviral (ARV), rilpivirine. Injectable CAB for PrEP is sometimes also referred to as long-acting CAB, or CAB-LA. While two months is considered long-acting for HIV prevention products, in the wider sexual and reproductive health sector, particularly family planning, two months is considered short-acting. For this reason, the term CAB for PrEP is used across PrEPWatch.
In December 2021, the FDA approved injectable CAB as a prevention option. ViiV’s list of registration efforts by country can be found at this link– scroll down to “Click here for the worldwide registration details of Cabotegravir PrEP.” And in July 2022, ViiV announced a voluntary licensing agreement with the Medicines Patent Pool and the WHO recommended CAB for PrEP as an additional prevention option, and released new guidelines for its use.
The next step is to plan for comprehensive, integrated access to this new addition to the prevention toolkit. AVAC’s Plan for Accelerating Access and Introduction of Injectable CAB for PrEP focuses on learning the lessons from the first ten years of delivering oral PrEP and how to move faster, more strategically, and with greater coordination to maximize the impact of CAB for PrEP. And AVAC’s Integrated Study Tracker consolidates information on all known CAB for PrEP implementation studies, where new evidence on effectively delivering CAB for PrEP will be generated, as well as modelling studies, clinical trials, open label extensions, and landscaping studies.
How It Works
Injectable PrEP inhibits HIV DNA from integrating with human DNA. Blocking this integration plays a role in both treatment and prevention. In treatment, combination injectable rilpivirine and cabotegravir can be used to maintain virologic suppression.
Injectable PrEP reduces the risk of acquiring HIV and is a longer-acting option compared to oral PrEP.
While daily oral PrEP is becoming more available and accessible across the world, multiple barriers affect adherence to daily pill-taking—from stigma and patriarchy to pill-size and side-effects. A longer acting option that eliminates the need for a daily pill may improve adherence for some.
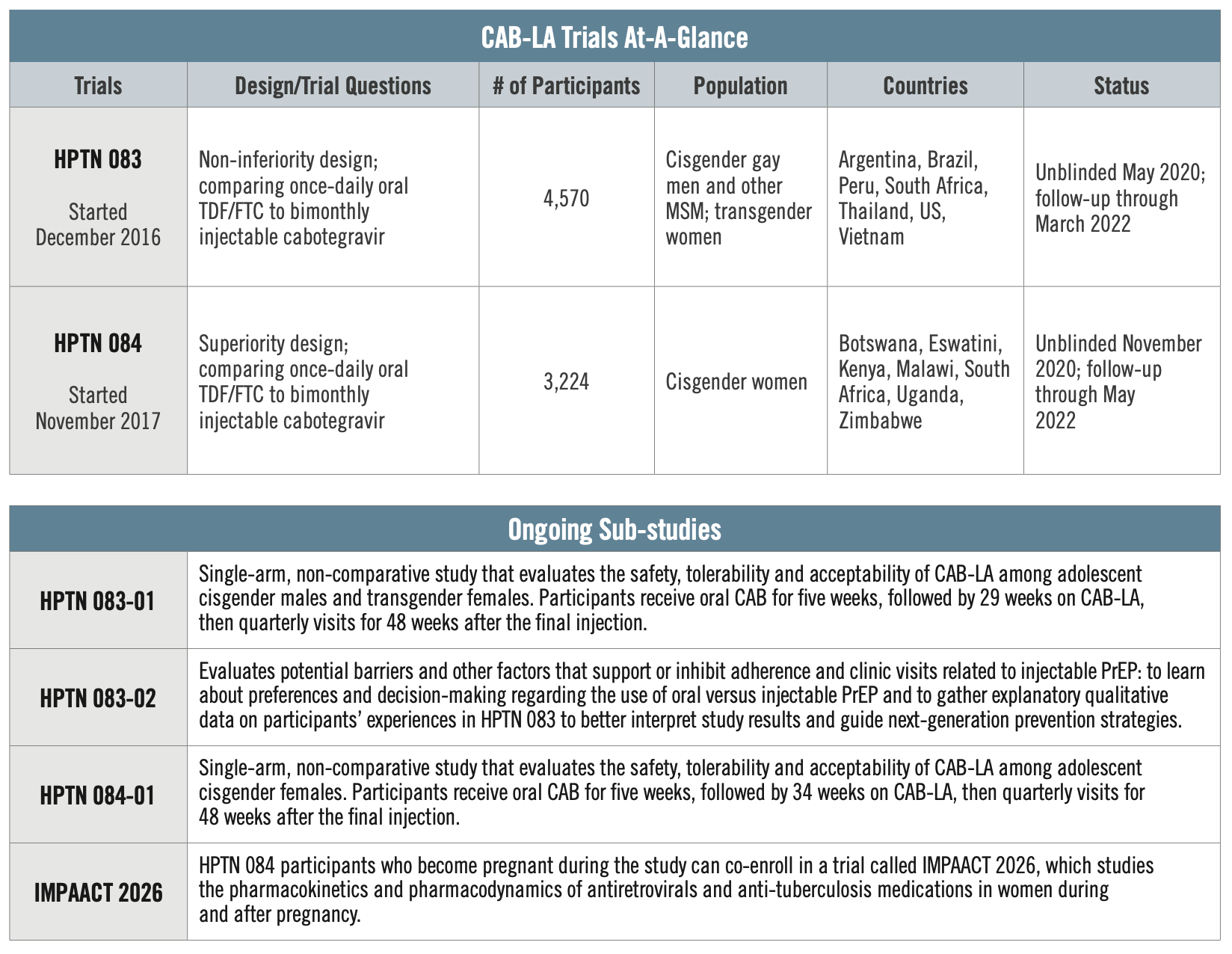
Data from both studies show injectable cabotegravir to be effective, safe, and well-tolerated in cisgender MSM, TGW, as well as cisgender women.
Like daily oral PrEP and the dapivirine vaginal ring (PrEP Ring), injectable PrEP does not protect against unintended pregnancies or STIs.
Injectable PrEP does not protect against other sexually transmitted infections (STIs) or unintended pregnancies. In fact, during the course of the trial, participants in both arms of HPTN 083 had high rates of other sexually transmitted infections, including new diagnoses of syphilis, chlamydia and gonorrhea. This is consistent with STI rates seen among oral PrEP users both inside and outside of clinical trials as well as with findings in HPTN 084.
These findings reinforce the need to deliver all PrEP strategies with comprehensive, integrated services that include counseling, basic healthcare, contraception and other sexual and reproductive health services, and linkages to HIV treatment and care as needed.
Potential Implementation Issues for injectable CAB for PrEP
Testing
Diagnostics currently used for HIV testing may not be sensitive enough to detect early HIV infection in people receiving injectable PrEP. As a result, people who acquire HIV close in time to initiating injectable PrEP may not test positive, increasing their risk of developing HIV that is resistant to treatment, though the documented numbers of people who have developed resistant HIV while taking injectable PrEP remain very small. More sophisticated and expensive diagnostics such as viral load testing could push the cost of delivering injectable PrEP beyond country budgets. Employing these diagnostics would also require additional training for providers and adaptations to the supply chain system.
Pharmacokinetic Tail
In addition, a decreasing amount of drug is present in the bloodstream, the pharmacokinetic tail, for a period of time after someone discontinues injections. Cabotegravir may not be present at protective levels during the tail. This could result in the development of drug resistant HIV following exposure. Both HPTN 083 and 084 are monitored closely for this effect, but the number of people who became HIV positive during the trials was so small that data is inconclusive on this question. It is not yet clear whether this long pharmacokinetic tail will have a significant impact on drug resistance.
Potential Access Barriers
Once injectable PrEP moves from open label extensions to implementation more will be known about the specific barriers to access. Several have been cited. These include: the site of administration (in the buttocks), which require a private room; dosing frequency of every two months, which does not align with most injectable contraceptive visits of every two to three months for many cisgender women in low and middle income countries; and having consistent access to a health facility to obtain the injection on a regular basis.
Pregnant and Lactating People
More research is needed to understand how CAB for PrEP functions in pregnant and lactating people and what impact it might have on a developing fetus. A number of participants became pregnant during HPTN 084. While no adverse effects were documented in either the parents or the infants, HPTN 084 was not designed to answer questions about pregnancy nor was it large enough to detect potentially adverse health effects on participants.
In the open label extension for HPTN 084, participants will no longer be offered contraception. If a participant becomes pregnant, they will be offered the choice of continuing CAB for PrEP. These results, once available will add to the body of knowledge about safety and efficacy of CAB in pregnant people. It is anticipated that any regulatory approvals and guidelines for use of injectable PrEP will include guidance for people who are pregnant or lactating.
Coalition to Accelerate Access to Long-Acting PrEP
Addressing these crucial issues around access to CAB for PrEP has implications for all future HIV prevention interventions. The Coalition to Accelerate Access to Long-Acting PrEP brings together leading donors, agencies, and advocates aimed at a practical goal: jointly develop strategies and take coordinated action to identify and overcome access challenges to just-approved and future PrEP options. AVAC serves as the Secretariat. Find more on the Coalition and its work on the Coalition’s project page on AVAC.org.
Evidence and Research for Injectable Cabotegravir (CAB) as PrEP
Clinical Trial Evidence
HPTN 083
Conducted by the HIV Prevention Trials Network, HPTN 083 is comparing CAB to daily oral PrEP. The study enrolled 4,570 cisgender MSM and TGW who have sex with men across 43 global sites in Argentina, Brazil, Peru, South Africa, Thailand, the US, and Vietnam. Researchers found about three times more incidence of HIV in the oral PrEP group than there were in the CAB for PrEP group. The study found risk of HIV reduced by 66 percent compared to those taking oral PrEP.
HPTN 084
Also conducted by the HIV Prevention Trials Network, HPTN 084, compares injectable CAB to daily oral PrEP among cisgender women. This study enrolled 3,224 women between the ages of 18 and 45, all of whom were at risk of acquiring HIV, from seven African countries, including Botswana, Eswatini, Kenya, Malawi, South Africa, Uganda and Zimbabwe. Researchers found that injectable CAB was both safe and statistically superior to daily oral PrEP in this population, with an 89 percent reduction in HIV incidence. Of 39 total HIV infections in the study, only four occurred among women who were receiving cabotegravir.
For more information see AVAC’s Advocates’ Primer on Long-Acting Injectable Cabotegravir for PrEP. For more on the design of these trials check out the podcast on Px Pulse, Testing Long-Acting PrEP, Easier Said Than Done.
Advocacy
Prevention options that mitigate or eliminate barriers to access are essential. Key populations, including men who have sex with men (MSM), transgender women (TGW), female sex workers (FSW), and adolescent girls and young women (AGYW) need effective options that work and fit into their lives. Each HIV prevention option has unique characteristics, and individuals may prefer a particular PrEP method for any number of reasons.
Ensuring informed choice is key. Injectable CAB for PrEP is another strategy to help reduce HIV risk. For some people it will be the right one; for others, daily oral PrEP, the dapivirine vaginal ring (the PrEP ring) or another non ARV-based approach will be right. Research and development have created new options; now advocacy is needed to make them viable choices for people who need and want them.
What can advocates do now? Check out AVAC’s Advocates Primer on CAB for PrEP for ways you can become engaged.
Resources
- CAB for PrEP for Advocates: Basics of injectable cabotegravir for HIV prevention (slide deck)
- Injectable PrEP Factsheet for Transgender and Gender-Nonbinary Advocates and Community Members in the Asia-Pacific Region
- Injectable PrEP Factsheet for MSM Advocates and Community Members in the Asia-Pacific Region
- CAB PrEP Compendium of End User Insights