The dapivirine vaginal ring (DVR) is the first long-acting, user-controlled, non-systemic, HIV prevention product to be approved. Below are details on DVR’s distinct characteristics, approval history, availability, safety and effectiveness. Evidence and resources for learning more are also provided.
The Basics
- A flexible silicone ring that is inserted in the vagina and slowly releases an antiretroviral (ARV) drug called dapivirine over the course of one month.
- First developed by the International Partnership for Microbicides (IPM) and since acquired by the Population Council in 2022.
- First approved in 2021 by the Medicines Control Authority of Zimbabwe.
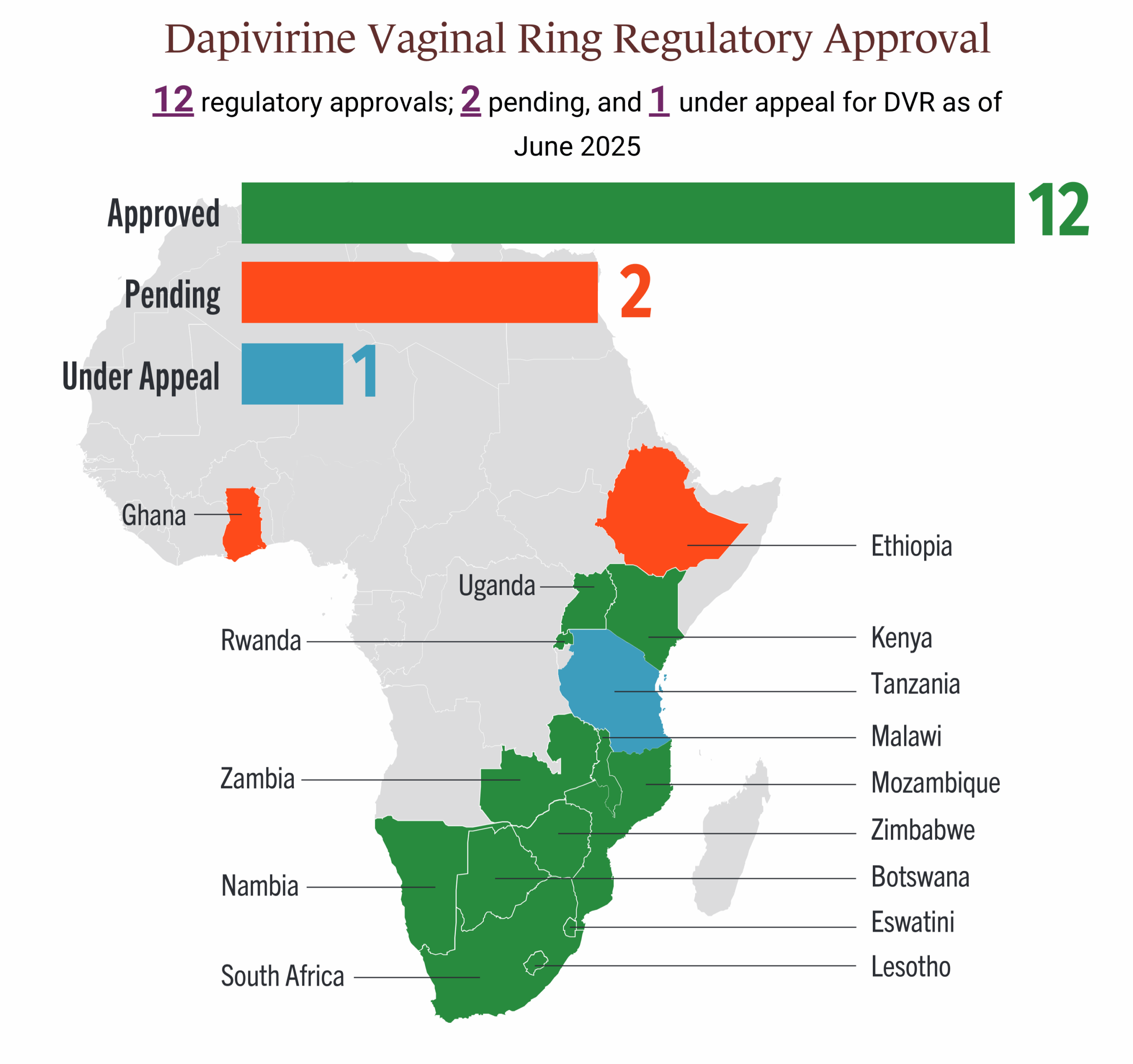
- Approved in several African countries—at this time it has not been submitted for approval outside of Africa.
- Not yet widely available outside of implementation studies.
- Only for use by people assigned female at birth.
- Brand name DapiRing- no generics currently available.
- User-controlled-can be inserted and removed by the user.
- A three-month version of the ring is currently under investigation. Early data has shown that it delivers dapivirine at higher levels than the one-month ring.
Effectiveness
- Topical drugs such as the DVR are locally-acting, which means the ring only protects against HIV through vaginal sex, in contrast to oral PrEP or injectable cabotegravir which act systemically and protect against transmission throughout the body.
- As a topical drug, very little dapivirine will be absorbed elsewhere in the body, making it unlikely to be found in high concentrations in the bloodstream and other body tissues. This may reduce side effects as well as the risk of development of HIV resistance.
- Studies show this locally acting option is a valuable choice for women seeking HIV prevention.
- Clinical trials showed 35% reduced risk of HIV infection, while later studies found risk reduced by as much as 50%-this difference could be due to higher adherence in later trials. See this summary of the research.
- The ring must be in place for 24 hours before it can reduce the risk of HIV infection.
- Only reduces the risk of HIV while it is in place-it should not be removed during sex, and is rarely felt by either partner.
- Must be replaced after one month.
- Does not prevent any sexually transmitted infections other than HIV.
- Does not prevent pregnancy, though multipurpose rings which also prevent pregnancy are currently being investigated in early stage clinical trials.
Safety
- Well-established tolerability with long-term use, including by adolescents.
- Data show no evidence the DVR increased resistance to treatment drugs that use the same class of ARVs, known as non-nucleoside reverse transcriptase inhibitors or NNRTIs.
- Favourable safety profile for pregnant and lactating people and their infants.
- Side effects can include vaginal discharge, itching, urinary tract infections, and pelvic and lower abdominal pain. In clinical trials, these were generally mild to moderate and resolved with no interruption in ring use.
- Safe to use with most forms of contraception, except for contraceptive vaginal rings, diaphragms, or cervical caps.
- Can be left in during menstruation and used with tampons, but not with menstrual cups.
Where can I access the DVR?
To see where DVR is currently approved and where approval is pending, see the graphic below or visit our Country Planning for Product Introduction Matrix. To find a provider near you, see our PrEP Access page. At this time, the DVR is not as widely available as oral PrEP, and is only available in a small number of locations and via implementation studies.
Evidence and Resources
Click here for an overview of DVR evidence and research to date, and click here for details of ongoing DVR implementation studies. Visit AVAC’s Global PrEP Tracker for the latest updates on PrEP uptake by country, including the DVR.
The MOSAIC project is accelerating the introduction and scale-up of new HIV prevention options for women, including the DVR. Click here for an overview of project tools and resources.
WHO’s Consolidated Guidelines on HIV Prevention, Testing, Treatment, Service Delivery and Monitoring brings together all the latest WHO recommendations on PrEP, including DVR.
Looking to roll out DVR in your country? The Plan 4 Ring toolkit guides implementers through the full planning process, and IPM’s Training Package can be used to train healthcare providers to deliver the DVR.
Looking to procure the DVR? The Global Fund and CIFF’s Early Market Access Vehicle (EMAV) is facilitating immediate access to the DVR to accelerate availability for users, expand PrEP choice, and catalyze impact while less expensive rings come to market with an approximate 60% drop in price/month.
See here for aidsmap’s comprehensive overview of the DVR.
For more DVR resources, see our resource library.